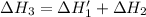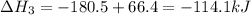Chemistry, 30.11.2019 01:31 tstaples02
Nitric acid can be manufactured in a multi-step process, during which nitric oxide is oxidized to create nitrogen dioxide. 2no (g) + o2 (g) → 2no2 (g)
calculate the standard reaction enthalpy for the above reaction using the following thermodynamic data.
n2 (g) + o2 (g) → 2no (g) ∆h˚1 = 180.5 kj
n2 (g) + 2o2 (g) → 2no2 (g) ∆h˚2 = 66.4 kj
-252.4 kj/mol rxn
-114.1 kj/mol rxn
-100.3 kj/mol rxn
-246.9 kj/mol rxn

Answers: 1


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 14:00, njones58emailtjcedu
What mass of natural gas (ch4) must you burn to emit 276 kj of heat?
Answers: 1

Chemistry, 22.06.2019 15:30, lizzyhearts
Count the number of each type of atom in the equation below, and then balance the equation. write in the numbers of atoms and coefficients. add a 1 if there should be no coefficient. cs2(l) + o2(g) → co2(g) + so2(g) c [ ] s [ ] o > c [ ] s [ ] o [ ] cs2(l) + [ ] o2(g) > [ ] co2(g) + [ ] so2(g)
Answers: 3

Chemistry, 22.06.2019 16:30, Kathryn014
4. a 20-kg child is tossed up into the air by her parent. the child is 2 meters off the ground traveling 5 m/s. circle one: ke / gpe / both show your work for finding the values of each type of energy the object has:
Answers: 1
You know the right answer?
Nitric acid can be manufactured in a multi-step process, during which nitric oxide is oxidized to cr...
Questions in other subjects:

Mathematics, 24.11.2020 22:40









 (1)
(1) 
 (2)
(2) 
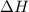 for the following reaction i.e,
for the following reaction i.e,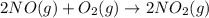 (3)
(3) 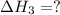
 (1')
(1') 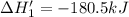
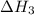 for the reaction will be:
for the reaction will be: