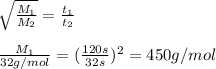Chemistry, 30.11.2019 01:31 hannahbeccahxo9681
Agas of unknown molecular mass was allowed to effuse through a small opening under constant-pressure conditions. it required 120 s for 1.0 l of the gas to effuse. under identical experimental conditions it required 32 s for 1.0 l of o2 gas to effuse. you may want to reference (pages 416 - 419) section 10.8 while completing this problem. part a calculate the molar mass of the unknown gas. (remember that the faster the rate of effusion, the shorter the time required for effusion of 1.0 l; that is, rate and time are inversely proportional.)

Answers: 3


Other questions on the subject: Chemistry



Chemistry, 21.06.2019 22:00, pollywallythecat
Your friend offers to show you an intrusive igneous rock. which of the following would you expect to see?
Answers: 1

Chemistry, 22.06.2019 15:30, sanchez7489
Draw the lewis dot structure for each of the following polyatomic ions
Answers: 1
You know the right answer?
Agas of unknown molecular mass was allowed to effuse through a small opening under constant-pressure...
Questions in other subjects:


Law, 24.11.2021 06:40


Mathematics, 24.11.2021 06:40

Biology, 24.11.2021 06:40


Mathematics, 24.11.2021 06:40