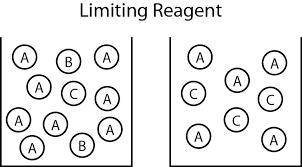
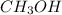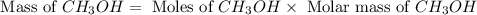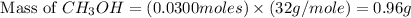Carbon monoxide gas reacts with hydrogen gas to form methanol via the following reaction: co(g)+2h2(g)→ch3oh(g) a 1.65 l reaction vessel, initially at 305 k, contains carbon monoxide gas at a partial pressure of 232 mmhg and hydrogen gas at a partial pressure of 374 mmhg .identify the limiting reactant and determine the theoretical yeild of methonal in grams.

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 14:30, lejeanjamespete1
Heat flow inside earth activity i chose chemistry cause there is no science alert! the newspaper has been hacked, and all of the headlines have been changed to reflect old legends. it is your task to make sure they are corrected. choose one of the newspaper headlines. you will rewrite the headline and a one-to-two-paragraph article to make the news scientifically correct. be sure to include the following in your corrected newspaper article: new title reflecting correct information detailed information about the processes and tectonic plate interactions that are causing the geological event to occur real-world example of where to find the geological event on earth (picture and location) also review the grading rubric before you begin.
Answers: 1

Chemistry, 21.06.2019 14:30, krystenlitten
Find the protons, electrons and neutrons for strontium with a mass of 83
Answers: 1

Chemistry, 22.06.2019 13:00, nadiarose6345
In a copper wire, a temperature increase is the result of which of the following
Answers: 1

You know the right answer?
Carbon monoxide gas reacts with hydrogen gas to form methanol via the following reaction: co(g)+2h2...
Questions in other subjects:



Spanish, 14.07.2020 23:01

Geography, 14.07.2020 23:01




Mathematics, 14.07.2020 23:01

Mathematics, 14.07.2020 23:01

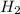 and the theoretical yield of methanol is, 0.96 grams.
and the theoretical yield of methanol is, 0.96 grams.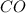 and
and 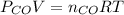
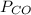 = pressure of CO gas = 232 mmHg = 0.305 atm (1 atm = 760 mmHg)
= pressure of CO gas = 232 mmHg = 0.305 atm (1 atm = 760 mmHg)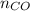 = number of moles of CO gas = ?
= number of moles of CO gas = ?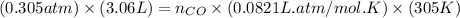
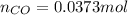
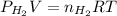
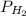 = pressure of
= pressure of 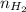 = number of moles of
= number of moles of 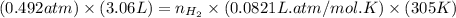
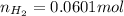
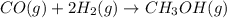
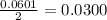 moles of
moles of