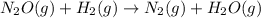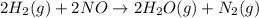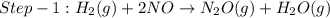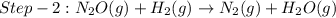Chemistry, 30.11.2019 01:31 uticabadgirl
The reduction of nitrogen monoxide is described by the following chemical equation: 2h2 (g) +2no (g) 2h20 ()n2 (g suppose a two-step mechanism is proposed for this reaction, beginning with this elementary reaction: h2 g+2no(g)- n20 (g)+h20(g) suppose also that the second step of the mechanism should be bimolecular suggest a reasonable second step. that is, write the balanced chemical equation of a bimolecular elementary reaction that would complete the proposed mechanism

Answers: 1


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 14:20, greenbyron88
Which statement explains why the bonds between non metals tend to be covalent? the bonds are found to be nondirectional they have large differences in electronegativity they have small differences in electronegativity they have ions that produce an electrostatic pull
Answers: 1


Chemistry, 23.06.2019 03:30, cupcake3103670
Name 3 types of energy you see being used as you look around a classroom
Answers: 1
You know the right answer?
The reduction of nitrogen monoxide is described by the following chemical equation: 2h2 (g) +2no (g...
Questions in other subjects:

Mathematics, 16.09.2020 09:01

Mathematics, 16.09.2020 09:01

Mathematics, 16.09.2020 09:01

Mathematics, 16.09.2020 09:01

Physics, 16.09.2020 09:01

Mathematics, 16.09.2020 09:01

Mathematics, 16.09.2020 09:01

Mathematics, 16.09.2020 09:01

Mathematics, 16.09.2020 09:01

English, 16.09.2020 09:01