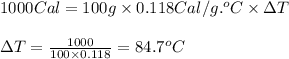The specific heats and densities of several materials are given below:
material specific heat...

The specific heats and densities of several materials are given below:
material specific heat (cal/g·°c) density (g/cm3)
brick 0.220 2.0
concrete 0.270 2.7
steel 0.118 7
water 1.00 1.00
calculate the change in temperature produced by the addition of 1 kcal of heat to 100 g of steel.
a. 84.7°c
b. 37.0°c
c. 1.43°c
d. 1.18°c

Answers: 3


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 02:00, bernicewhite156
Will give brainliest it is a lab from k12 here is the linkfor each metal that participated in a chemical change, write the type of metal it is, based on your examination of the periodic table. type your answer here. (score for question 3: of 5 points) were there any metallic compounds that did not react with either the acid or the base? write the type of metal, based on your examination of the periodic table. type your answer here. (score for question 4: of 5 points) make a general statement about the reactivity of the metals in this experiment. type your answer here.
Answers: 2

Chemistry, 22.06.2019 02:50, JuniperGalaxy
Consider the equilibrium system: 2icl(s) ⇄ i2(s) + cl2(g) which of the following changes will increase the total amount of of cl2 that can be produced? all of the listed answers are correct decreasing the volume of the container removing the cl2 as it is formed adding more icl(s) removing some of the i2(s)
Answers: 1

Chemistry, 22.06.2019 07:20, mathman783
Why does his teacher ask him to balance the equation by including the correct coefficient
Answers: 1
You know the right answer?
Questions in other subjects:


English, 06.09.2020 03:01

Mathematics, 06.09.2020 03:01



Mathematics, 06.09.2020 03:01

Mathematics, 06.09.2020 03:01

Health, 06.09.2020 03:01


Social Studies, 06.09.2020 03:01

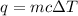
 = change in temperature = ?
= change in temperature = ?