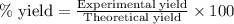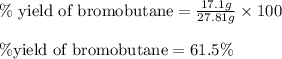Chemistry, 30.11.2019 01:31 akatsionis25
Use the following reaction: c4h9oh + nabr + h2so4 c4h9br + nahso4 + h2o if 15.0 g of c4h9oh react with 22.4 g of nabr and 32.7 g of h2so4 to yield 17.1 g of c4h9br, what is the percent yield of this reaction? remember: percent yield is your (experimental yield/theoretical yield)x100.

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 04:30, EinsteinBro
Which statement best describes the relationship between period and frequency of light waves? a) in wave b the period increases and the frequency decreases from wave a. b) in wave a the period increases and the frequency decreases from wave b. c) in wave b the period is shorter and the frequency is greater than in wave a. d) in wave a the period is shorter and the frequency is greater than in wave b.
Answers: 1

Chemistry, 22.06.2019 08:00, PrincessKeliah5538
Me i dont know what to do! the table compares the number of electrons in two unknown neutral atoms. comparison of electrons atom number of electrons a 10 d 11 use this information to determine the number of valence electrons in the atoms. which of the following correctly compares the stability of the two atoms? both are unreactive. both are highly reactive. a is unreactive and d is reactive. a is reactive and d is unreactive.
Answers: 1

Chemistry, 23.06.2019 03:10, 3jazybraxy
Which is true according to the law of conservation of energy
Answers: 1

Chemistry, 23.06.2019 09:00, payshencec21
How many grams of ammonia are produced when 1.0 mole of nitrogen reacts
Answers: 2
You know the right answer?
Use the following reaction: c4h9oh + nabr + h2so4 c4h9br + nahso4 + h2o if 15.0 g of c4h9oh react w...
Questions in other subjects:



Mathematics, 19.06.2020 17:57



Mathematics, 19.06.2020 17:57


Mathematics, 19.06.2020 17:57


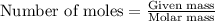 .....(1)
.....(1)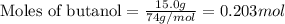
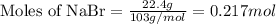
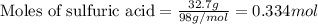
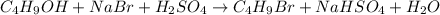
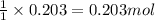 of bromobutane
of bromobutane