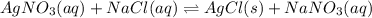Answers: 2


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 14:30, lejeanjamespete1
Heat flow inside earth activity i chose chemistry cause there is no science alert! the newspaper has been hacked, and all of the headlines have been changed to reflect old legends. it is your task to make sure they are corrected. choose one of the newspaper headlines. you will rewrite the headline and a one-to-two-paragraph article to make the news scientifically correct. be sure to include the following in your corrected newspaper article: new title reflecting correct information detailed information about the processes and tectonic plate interactions that are causing the geological event to occur real-world example of where to find the geological event on earth (picture and location) also review the grading rubric before you begin.
Answers: 1

Chemistry, 22.06.2019 00:50, justabeachbum
If a reactant was removed, did the new equilibrium system shift to make more reactants or more products?
Answers: 1


Chemistry, 22.06.2019 11:00, peternice2956
Ais a mountain created from eruptions of lava, ash, rocks, and hot gases.
Answers: 1
You know the right answer?
For the endothermic reaction below, using lechatlier's principle, predict which way the equilibrium...
Questions in other subjects:







English, 01.09.2019 03:20

English, 01.09.2019 03:20

Chemistry, 01.09.2019 03:20