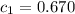Answers: 1


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 01:40, natannale
Darla claims that the first periodic table developed by mendeleev was not completely accurate, so it is not useful at all. harmony argues that it establish the periodic table we use today, making it more credible. who is correct and why? darla is correct, because a model that has any mistakes should be thrown out. darla is correct, because a good model would not need to change. harmony is correct, because mendeleev’s model had all of the information correct in the first version. harmony is correct, because mendeleev’s model made predictions that came true.
Answers: 1

Chemistry, 22.06.2019 02:20, ayoismeisalex
Brainliesttt me asap! the below reaction can be categorized as more than one type of reaction. which reactions are these, and what are the types of reactions?
Answers: 1

Chemistry, 22.06.2019 05:30, ayoismeisjjjjuan
What type of reaction is shown below? check all that apply. 2h2o2 → 2h2o + o2 synthesis decomposition combustion
Answers: 1
You know the right answer?
When a 40.0 g sample of a metal at 25.00 °c is added to 65.0 g of water at 100.00 °c, the final temp...
Questions in other subjects:

Mathematics, 29.09.2019 15:50

Mathematics, 29.09.2019 15:50

Mathematics, 29.09.2019 15:50




Mathematics, 29.09.2019 15:50

Biology, 29.09.2019 15:50


Chemistry, 29.09.2019 15:50


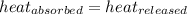
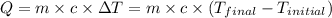
![m_1\times c_1\times (T_{final}-T_1)=-[m_2\times c_2\times (T_{final}-T_2)]](/tpl/images/0396/4522/09236.png) .................(1)
.................(1)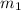 = mass of metal = 40.0 g
= mass of metal = 40.0 g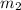 = mass of water = 65.0 g
= mass of water = 65.0 g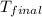 = final temperature =
= final temperature = 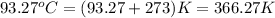
 = temperature of metal =
= temperature of metal = 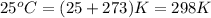
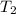 = temperature of water =
= temperature of water = 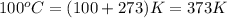
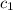 = specific heat of metal = ?
= specific heat of metal = ?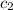 = specific heat of water=
= specific heat of water= 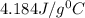
![40.0\times c_1\times (366.27-298)=-[65.0\times 4.184\times (366.27-373)]](/tpl/images/0396/4522/88e12.png)