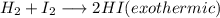Chemistry, 29.11.2019 07:31 heathkid23
 + i_2(g) \longrightarrow 2hi(g))
the forward reaction above is exothermic. at equilibrium, what happens if the reaction mixture is cooled at constant volume?
select all that apply.
1. the reaction absorbs energy.
2. the reaction releases energy.
3. [h₂] and [i₂] increase.
4. [h₂] and [i₂] decrease.
5. [h₂] and [i₂] remain constant.
6. [hi] increases.
7. [hi] decreases.
8. [hi] remains constant.
if c is added to the equilibrium system above, in which direction will the equilibrium shift?

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 00:30, mannster03
This active feature of earth's crust in building mountain ranges as well as islands. this feature is a a) cavern. b) earthquake. c) mountain. d) volcano.
Answers: 2

Chemistry, 22.06.2019 04:00, eborkins
Seltzer water is created by placing water under pressure with carbon dioxide gas. which of the following statements best describe seltzer water: a. the solution will be slightly acidic b. the solution will be slightly basic. the solution will be strongly acidic. d. the solution will be strongly basic. e. the solution will be neutral
Answers: 3


Chemistry, 22.06.2019 21:00, ciel8809
Which answer tells the reason the earth’s climate is getting warmer? too many animals are becoming extinct. large glaciers are melting in antarctica. the earth is moving closer to the sun. driving cars gives off gases that trap heat in the atmosphere.
Answers: 1
You know the right answer?
[tex]h_2(g) + i_2(g) \longrightarrow 2hi(g)[/tex]
the forward reaction above is exothermic. at...
the forward reaction above is exothermic. at...
Questions in other subjects:


Mathematics, 05.11.2020 01:00

History, 05.11.2020 01:00




English, 05.11.2020 01:00

Biology, 05.11.2020 01:00


![[H_{2}]](/tpl/images/0395/9023/f6f78.png) and
and ![[I_{2}]](/tpl/images/0395/9023/d0626.png) increase.
increase.![[HI]](/tpl/images/0395/9023/7d1e5.png) increases.
increases.