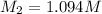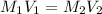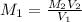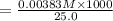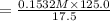Chemistry, 29.11.2019 06:31 sarahjdeering
A17.55 ml solution of potassium nitrate (kno3) was diluted to 125.0 ml, and 25.00 ml of this solution was then diluted to 1.000 × 103 ml. the concentration of the final solution is 0.00383 m. calculate the concentration of the original solution.

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 13:20, valencial0917
Why is an elements atomic mass not listed as a whole number on the periodic table
Answers: 2


Chemistry, 22.06.2019 00:30, timiaparker
What does x represent in the formula for the compound xcl4?
Answers: 2

Chemistry, 22.06.2019 07:20, camillexv2668
2pos suppose an object in free fall is dropped from a building. its starting velocity is 0 m/s. ignoring the effects of air resistance, what is the speed (in m/s) of the object after falling 3 seconds? give your answer as a positive decimal without units. answer here
Answers: 1
You know the right answer?
A17.55 ml solution of potassium nitrate (kno3) was diluted to 125.0 ml, and 25.00 ml of this solutio...
Questions in other subjects:

History, 18.11.2019 02:31

English, 18.11.2019 02:31

Business, 18.11.2019 02:31



Mathematics, 18.11.2019 02:31

Physics, 18.11.2019 02:31



Biology, 18.11.2019 02:31