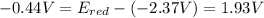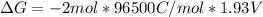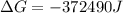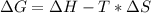Chemistry, 29.11.2019 06:31 Supermate11
Free-energy change, δg∘, is related to cell potential, e∘, by the equationδg∘=−nfe∘where n is the number of moles of electrons transferred and f=96,500c/(mol e−) is the faraday constant. when e∘ is measured in volts, δg∘ must be in joules since 1 j=1 c⋅v.1. calculate the standard free-energy change at 25 ∘c for the following reaction: mg(s)+fe2+(aq)→mg2+(aq)+fe(s)expres s your answer to three significant figures and include the appropriate units.2. calculate the standard cell potential at 25 ∘c for the reactionx(s)+2y+(aq)→x2+(aq)+2y(s)w here δh∘ = -675 kj and δs∘ = -357 j/k .express your answer to three significant figures and include the appropriate units.

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 13:30, kellywelly82
A48 g piece of ice at 0.0 ∘c is added to a sample of water at 7.4 ∘c. all of the ice melts and the temperature of the water decreases to 0.0 ∘c. how many grams of water were in the sample?
Answers: 1

Chemistry, 21.06.2019 13:30, dlshadowmorfe
Which of these best describes the scientific process
Answers: 3

Chemistry, 22.06.2019 19:10, krisandlance
Astudent completes a titration by adding 12.0 milliliters of naoh(aq) of unknown concentration to 16.0 milliliters of 0.15 m hcl(aq). what is the molar concentration of the naoh(aq)? 1)5.0 m 2)0.20 m 3)0.11 m 4)1.1 m
Answers: 1

Chemistry, 23.06.2019 03:00, KindaSmartPersonn
In which of the following phases of matter do molecules have the highest amount of energy? a. liquid b. gel c. solid d. gas
Answers: 2
You know the right answer?
Free-energy change, δg∘, is related to cell potential, e∘, by the equationδg∘=−nfe∘where n is the nu...
Questions in other subjects:


World Languages, 20.09.2020 07:01


Health, 20.09.2020 07:01

Social Studies, 20.09.2020 07:01





Biology, 20.09.2020 07:01

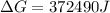
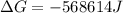
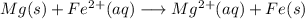
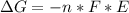
![E=E_{red}-E_{ox]](/tpl/images/0395/8514/34231.png)