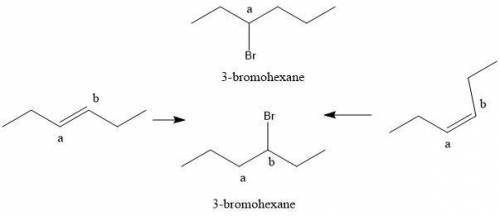
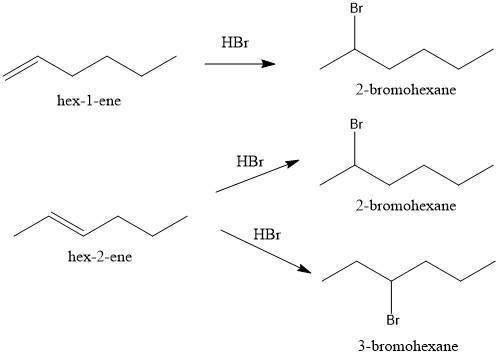
Compounds x and y are stereoisomers having the formula c6h12. both x and y react with one molar equivalent of hydrogen in the presence of a palladium catalyst to form hexane, and they each react with hbr to give a single bromoalkane product. draw structural formulas for both x and y.

Answers: 3


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 16:00, corcoranrobert1959
How do dying stars contribute to the formation of planets
Answers: 1

Chemistry, 22.06.2019 17:40, aaliyahthomas37
Which statement about hf is true? it is zero for any compound in its standard state. it is positive when the bonds of the product store more energy than those of the reactants. it is negative when a compound forms from elements in their standard states. it is zero for any element that is in the liquid state.
Answers: 1
You know the right answer?
Compounds x and y are stereoisomers having the formula c6h12. both x and y react with one molar equi...
Questions in other subjects:


Social Studies, 02.06.2021 17:30

Mathematics, 02.06.2021 17:30

Mathematics, 02.06.2021 17:30

Mathematics, 02.06.2021 17:30




Biology, 02.06.2021 17:30