
Chemistry, 29.11.2019 02:31 mathman783
Given that δ h ∘ f [ br ( g ) ] = 111.9 kj ⋅ mol − 1 δ h ∘ f [ c ( g ) ] = 716.7 kj ⋅ mol − 1 δ h ∘ f [ cbr 4 ( g ) ] = 29.4 kj ⋅ mol − 1 calculate the average molar bond enthalpy of the carbon‑bromine bond in a cbr 4 molecule.

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 02:20, kristieroth1
Compared with the freezing-point depression of a 0.01 m c6h12o6 solution, the freezing-point depression of a 0.01 m nacl solution is
Answers: 1

Chemistry, 22.06.2019 07:00, ceeejay0621
What is the main purpose of patent attorneys? defend the company against legal claims manage financial investments invent new products protect rights to new products and processes
Answers: 1

Chemistry, 22.06.2019 11:00, snowprincess99447
Which statement is true about hcl? (5 points) select one: a. it is a salt because it increases the concentration of metallic ions. b. it is a salt because it is formed by the reaction of an acid and a base. c. it is an acid because it increases the concentration of hydroxyl ions. d. it is an acid because it increases the concentration of hydronium ions.
Answers: 1

Chemistry, 22.06.2019 23:20, svaskeacevilles5477
In medium-sized stars such as the sun, nuclear fusion almost always means the fusing of nuclei to form , but larger stars can produce elements as heavy as
Answers: 2
You know the right answer?
Given that δ h ∘ f [ br ( g ) ] = 111.9 kj ⋅ mol − 1 δ h ∘ f [ c ( g ) ] = 716.7 kj ⋅ mol − 1 δ h ∘...
Questions in other subjects:







Social Studies, 01.12.2020 21:30

Business, 01.12.2020 21:30


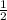 Br2(g) ⇒ Br(g) , Δ H ∘ = 111.9 kJ ⋅ mol − 1
Br2(g) ⇒ Br(g) , Δ H ∘ = 111.9 kJ ⋅ mol − 1 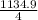 = 283.725 kJ ⋅ mol − 1
= 283.725 kJ ⋅ mol − 1

