Answers: 3


Other questions on the subject: Chemistry



Chemistry, 22.06.2019 22:40, destineysarah
Covalent bonds generally form when the bonded elements have a difference in electronegativity less than 1.5. subtract the electronegativities for the following pairs of elements and predict whether they form a covalent bond. electronegativity difference of c and c: ionic covalent electronegativity difference of mg and cl: ionic covalent
Answers: 1

Chemistry, 23.06.2019 02:20, theactualslash
In a chemical reaction, the final amount of the products is determined by the a. universal gas law b. law of definite proportions c. air pressure d. temperature e. none of the above me
Answers: 2
You know the right answer?
40.00 ml of 0.10 m koh solution is titrated by 30.00 ml of 0.10 m hcl (adding hcl to koh). what is t...
Questions in other subjects:

Mathematics, 16.07.2019 09:00



Mathematics, 16.07.2019 09:00

Mathematics, 16.07.2019 09:00

Mathematics, 16.07.2019 09:00



Mathematics, 16.07.2019 09:00

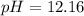
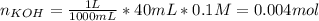
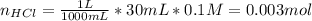

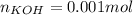
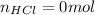
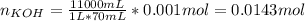
![pOH=-log([OH^-])=-log(0.0143)=1.84](/tpl/images/0395/5402/b4673.png)