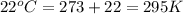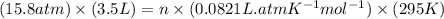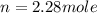Chemistry, 28.11.2019 21:31 carleenespriu
How many moles of gas must be forced into a 3.5 l ball to give it a gauge pressure of 8.8 psi at 22 ∘c? the gauge pressure is relative to atmospheric pressure. assume that atmospheric pressure is 14.5 psi so that the total pressure in the ball is 23.3 psi .

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 00:30, BLASIANNkidd
Maria wants to determine which type of disinfectant kills the most bacteria. which of the following is the best way for maria to determine this? a. ask ten different companies that make disinfectants which type is best. b. put the same amount and species of bacteria on ten identical plates, and add ten different kinds of disinfectant to each plate. c. interview ten different people to determine which type of disinfectant they prefer. d. put the same amount and species of bacteria on ten identical plates, and add a different disinfectant to each plate.
Answers: 1

Chemistry, 22.06.2019 18:00, jalenclarke25
What volume would 2.25 moles of ne has occupy at stp?
Answers: 1


Chemistry, 23.06.2019 12:30, lindseylewis313
When utilizing a transmission electron microscope, why is it necessary to stain the specimen with heavy metal salts?
Answers: 1
You know the right answer?
How many moles of gas must be forced into a 3.5 l ball to give it a gauge pressure of 8.8 psi at 22...
Questions in other subjects:

Business, 13.11.2020 07:20



History, 13.11.2020 07:20

Biology, 13.11.2020 07:20

Mathematics, 13.11.2020 07:20