

Answers: 1


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 09:30, kevinh2683
Apump contains 0.5 l of air at 203 kpa. you draw back on the piston of the pump, expanding the volume until the pressure reads 50.8 kpa. what is the new volume of the air pump
Answers: 2

Chemistry, 22.06.2019 17:30, mwest200316
To find the enthalpy of a reaction in the lab, you measured the of the reactants and the change during the reaction.
Answers: 1

Chemistry, 23.06.2019 01:30, nikonee
Astudent states that 9.0 g of baking soda will form an unsaturated solution in 100 g of water. what do you need to know to decide whether this statement is correct? a. the temperature of the water and the molar mass of baking soda b. the percent by volume of the solution and the solubility of baking soda c. the temperature of the water and the solubility of baking soda at that temperature
Answers: 1
You know the right answer?
When aluminum is placed in concentrated hydrochloric acid, hydrogen gas is produced. 2 al ( s ) + 6...
Questions in other subjects:



Mathematics, 01.09.2019 01:30

Business, 01.09.2019 01:30


Biology, 01.09.2019 01:30




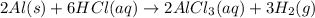

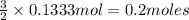 of hydrogen gas
of hydrogen gas


