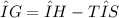Which of the following is true?
a. a reaction in which the entropy of the system increases ca...

Which of the following is true?
a. a reaction in which the entropy of the system increases can be spontaneous only if it is endothermic.
b. a reaction in which the entropy of the system decreases can be spontaneous only if it is endothermic.
c. a reaction in which the entropy of the system decreases can be spontaneous only if it is exothermic.
d. a reaction in which the entropy of the system increases can be spontaneous only if it is exothermic.

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 01:30, adrian08022
When an object falls through the air and encounters air resistance its overall speed will be than if it had not encountered air resistance? (one word answer)
Answers: 2

Chemistry, 22.06.2019 19:00, innocentman69
How does a catalyst increase the speed of a reaction? a. the catalyst eliminates the activated complex stage, allowing products to form immediately. b. the catalyst lowers the energy level of the reactants, making it easier for them to react. c. the catalyst makes it easier for the activated complex to form, lowering the activation energy. d. the catalyst raises the energy level of the products, making the reaction finish sooner. reset next
Answers: 1


Chemistry, 23.06.2019 09:20, goldwinner300
Asolution of naoh has a concentration of 25.00% by mass. what mass of naoh is present in 0.250 g of this solution? use the periodic table in the toolbar if needed. 0.0625 g what mass of naoh must be added to the solution to increase the concentration to 30.00% by mass? g
Answers: 2
You know the right answer?
Questions in other subjects:



Mathematics, 22.04.2020 17:22

Mathematics, 22.04.2020 17:22

Geography, 22.04.2020 17:22

Mathematics, 22.04.2020 17:22