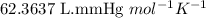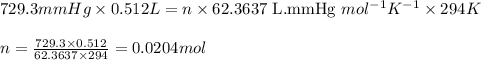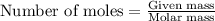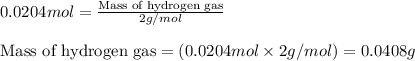Chemistry, 28.11.2019 19:31 snowprincess99447
Asample of hydrogen gas was collected over water at 21°c and at a pressure equal to the atmospheric pressure of 748 mm hg. the volume of the sample was 512 ml. how many grams of h2 are in the sample? the vapor pressure of water at 21°c is 18.7 mmhg.

Answers: 2


Other questions on the subject: Chemistry



Chemistry, 22.06.2019 10:00, halohero7
3. how much energy in joules is required to evaporate .0005 kg of liquid ammonia to vapor at the same temperature? 4. how much energy ( in megajoules ) is given up by .75 kg of water at 0c when it freezes to form ice at 0c? 5. explain how heat works between and at critical temperatures?
Answers: 2

Chemistry, 22.06.2019 23:30, bxymichelle
With the largest atoms and the smallest number of valence electrons and with the smallest atoms and the greatest number of valence electrons are the most reactive. a. nonmetals; metals b. nonmetals; transition elements c. transition elements; metals d. metals; nonmetals
Answers: 3
You know the right answer?
Asample of hydrogen gas was collected over water at 21°c and at a pressure equal to the atmospheric...
Questions in other subjects:

English, 26.08.2021 04:30

Mathematics, 26.08.2021 04:30

Mathematics, 26.08.2021 04:30

History, 26.08.2021 04:30

History, 26.08.2021 04:30

Mathematics, 26.08.2021 04:30



Chemistry, 26.08.2021 04:30

![21^oC=[21+273]K=294\ K](/tpl/images/0394/9433/09af2.png)