
Chemistry, 28.11.2019 06:31 mxltie1651
A9.000l tank at 27.0°c is filled with 5.29g of sulfur tetrafluoride gas and 15.6g of carbon dioxide gas. you can assume both gases behave as ideal gases under these conditions. calculate the mole fraction and partial pressure of each gas, and the total pressure in the tank. round each of your answers to 3 significant digits. sulfur tetraflouride: mole fraction? partial pressure? carbon dioxide: mole fraction? partial pressure? total pressure in tank?

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 01:20, whrjegt4jrnfdvj
1. suppose a reaction mixture, when diluted with water, afforded 300 ml of an aqueous solution of 30 g of the reaction product malononitrile [ch2(cn)2], which is to be isolated by extraction with ether. the solubility of malononitrile in ether at room temperature is 20.0 g/100 ml, and in water is 13.3 g/100 ml. what weight of malononitrile would be recovered by extraction with (a) three 100-ml portions of ether and (b) one 300-ml portion of ether? suggestion: for each extraction, let x equal the weight extracted into the ether layer. in part (a), the concentration in the ether layer is x/100 and in the water layer is (30 x)/300; the ratio of these quantities is equal to k 20/13.3.
Answers: 2

Chemistry, 22.06.2019 04:00, fantasticratz2
Acontainer holds 35.8 moles of gas under 10.0 atm of pressure at 70.0 c. what is the volume of the container?
Answers: 2

Chemistry, 22.06.2019 21:00, agarcia24101993
What is the chemical formula for the compound formed between sodium and flour one
Answers: 1

Chemistry, 22.06.2019 21:30, liamgreene90
In one or two grammatically correct sentences, write a definition for the term molecule geometry
Answers: 3
You know the right answer?
A9.000l tank at 27.0°c is filled with 5.29g of sulfur tetrafluoride gas and 15.6g of carbon dioxide...
Questions in other subjects:


Mathematics, 04.03.2022 14:00


Mathematics, 04.03.2022 14:00


Chemistry, 04.03.2022 14:00


Mathematics, 04.03.2022 14:00

Chemistry, 04.03.2022 14:00

Biology, 04.03.2022 14:00

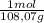 = 0,0489 moles SF₄
= 0,0489 moles SF₄ = 0,354 moles CO₂
= 0,354 moles CO₂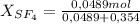 = 0,121
= 0,121 = 0,879
= 0,879

