
Chemistry, 28.11.2019 06:31 bernadetteindre6650
Calculate the ph of the solution formed when 45.0 ml of 0.100m naoh solution is added to 50.0 ml of 0.100m ch3cooh (ka for acetic acid = 1.8 x10-5 ).

Answers: 2


Other questions on the subject: Chemistry




Chemistry, 22.06.2019 14:10, cameronbeaugh
13. a covalent bond between two atoms is likely to be polar if: a. one of the atoms is much more electronegative than the other. b. the two atoms are equally electronegative. c. the two atoms are of the same element. d. the bond is part of a tetrahedrally shaped molecule. e. one atom is an anion.
Answers: 1
You know the right answer?
Calculate the ph of the solution formed when 45.0 ml of 0.100m naoh solution is added to 50.0 ml of...
Questions in other subjects:


Mathematics, 20.08.2019 13:10

Physics, 20.08.2019 13:10


Mathematics, 20.08.2019 13:10

Mathematics, 20.08.2019 13:10

Mathematics, 20.08.2019 13:10

English, 20.08.2019 13:10


![pH=pKa+log(\frac{[salt]}{[acid]} )](/tpl/images/0394/4621/dd797.png)
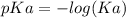
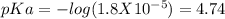
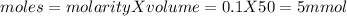
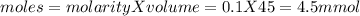
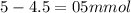
![pH=pKa+log(\frac{[salt]}{[acid]} )=4.74+log(\frac{4.5}{0.5})=5.69](/tpl/images/0394/4621/b17c8.png)


