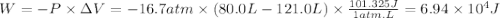Chemistry, 28.11.2019 05:31 loraine4664
Calculate the work associated with the compression of a gas from 121.0 l to 80.0 l at a constant pressure of 16.7 atm.

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 11:00, Usman458
The twister and runaway train are two coasters at the same amusement park. both coasters start at the same height. the coaster for the twister is twice the mass of the coaster for the runaway train. which roller coaster has greater gravitational potential energy at the start of the ride?
Answers: 1



Chemistry, 22.06.2019 23:00, SophieCasey
What is the oxidation state of each individual carbon atom in c2o42−?
Answers: 1
You know the right answer?
Calculate the work associated with the compression of a gas from 121.0 l to 80.0 l at a constant pre...
Questions in other subjects:


Mathematics, 04.05.2020 23:52


Mathematics, 04.05.2020 23:52



Mathematics, 04.05.2020 23:52

Mathematics, 04.05.2020 23:52


Mathematics, 04.05.2020 23:52