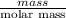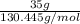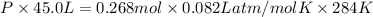Chemistry, 28.11.2019 05:31 cookies1164
Areaction between liquid reactants takes place at - 11.0 degree c in a sealed, evacuated vessel with a measured volume of 45.0 l. measurements show that the reaction produced 35. g of chlorine pentafluoride gas. calculate the pressure of chlorine pentafluoride gas in the reaction vessel after the reaction. you may ignore the volume of the liquid of reactants. be sure your answer has the correct number of significant digits.

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 19:00, RedDemon59
Apeak with a retention time of 407 s has a width at half-height (w1/2) of 7.6 s. a neighboring peak is eluted 17 s later with a w1/2 of 9.4 s. a compound that is known not to be retained was eluted in 2.5 s. the peaks are not baseline resolved. how many theoretical plates would be needed to achieve a resolution of 1.5?
Answers: 2

Chemistry, 22.06.2019 15:00, emmalie52
Phosphorous acid, h3po3(aq) , is a diprotic oxyacid that is an important compound in industry and agriculture. p k a1 p k a2 1.30 6.70 calculate the ph for each of the points in the titration of 50.0 ml of 1.5 m h3po3(aq) 1.5 m h 3 po 3 ( aq ) with 1.5 m koh(aq). 1.5 m koh ( aq ) .
Answers: 1


Chemistry, 22.06.2019 19:00, HaydenSturgis1
Which is the solubility product expression for caf2(s)?  [ca2+]/[f–]2  [ca2+][f2–]  [ca]+[f]2  [ca2+][f–]2
Answers: 3
You know the right answer?
Areaction between liquid reactants takes place at - 11.0 degree c in a sealed, evacuated vessel with...
Questions in other subjects:

Mathematics, 20.08.2019 12:00

Mathematics, 20.08.2019 12:00

History, 20.08.2019 12:00



Mathematics, 20.08.2019 12:00


Mathematics, 20.08.2019 12:00

Mathematics, 20.08.2019 12:00

History, 20.08.2019 12:00

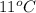 = (11 + 273) K = 284 K, V = 45.0 L
= (11 + 273) K = 284 K, V = 45.0 L