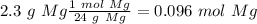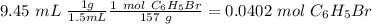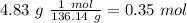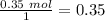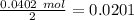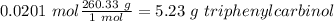Chemistry, 28.11.2019 04:31 kps26pd2mea
After distilling your crude methyl benzoate, you set aside 4.83 grams of the purified ester. you then prepare the grignard reagent ( phenylmagnesium bromide ) by reacting 2.3 grams of magnesium with 9.45 ml of bromobenzene. you add the 4.83 grams of methyl benzoate to the freshly prepared grignard reagent to form an addition product. finally, after hydrolyzing the grignard addition product, you obtain 5 grams of the final product, triphenyl carbinol. what is the percent yield of triphenyl carbinol?

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 00:20, TamB01
Use the gizmo to find the concentration of the mystery ch3cooh. use the titrant and indicator shown below perform the titration. what is the titrant volume? titrant analyte indicator titrant volume analyte concentration naoh ch3cooh phenophthalein select one: a. 20.0 ml b. 27.0 ml c. 30.0 ml d. 24.0 ml
Answers: 2

Chemistry, 22.06.2019 08:30, Blaise2653
Joan writes four numbers on the board in standard form, and then she writes their scientific notation
Answers: 1


Chemistry, 22.06.2019 22:30, angelagonzalesownus1
Which statement best summarizes the importance of ernest rutherford’s gold foil experiment? it proved that all of john dalton’s postulates were true. it verified j. j. thomson’s work on the atomic structure. it showed that an electron circles a nucleus in a fixed-energy orbit. it showed that a nucleus occupies a small part of the whole atom.
Answers: 1
You know the right answer?
After distilling your crude methyl benzoate, you set aside 4.83 grams of the purified ester. you the...
Questions in other subjects:



Biology, 23.07.2019 14:50


English, 23.07.2019 14:50

Mathematics, 23.07.2019 14:50


Geography, 23.07.2019 14:50

Mathematics, 23.07.2019 14:50

Health, 23.07.2019 14:50

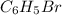 )= 157 g/mol
)= 157 g/mol