
Chemistry, 28.11.2019 04:31 dbn4everloved8
A1.00 g sample of n-hexane (c6h14) undergoes complete combustion with excess o2 in a bomb calorimeter. the temperature of the 1502 g of water surrounding the bomb rises from 22.64°c to 29.30°c. the heat capacity of the hardware component of the calorimeter (everything that is not water) is 4042 j/°c. what is δu for the combustion of n-c6h14? one mole of n-c6h14 is 86.1 g. the specific heat of water is 4.184 j/g·°c.

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 05:30, nuclearfire278
Why is soap used to remove grease? a. its nonpolar end dissolves the grease. b. it makes the water bond with the grease. c. it chemically bonds with the grease. d. its polar end dissolves the grease. correct answer for apex - a, its nonpolar end dissolves the grease.
Answers: 1


Chemistry, 22.06.2019 22:00, notearslefttocry14
Imagine one batch of soup (batch “a”) is made with 8.19 g/can of salt, according to the recipe, and a second batch of soup (batch “b”) is made with 8.32 g/can of salt. explain which batch would be more resistant to frost damage if it is shipped a great distance in winter and explain why.
Answers: 2
You know the right answer?
A1.00 g sample of n-hexane (c6h14) undergoes complete combustion with excess o2 in a bomb calorimete...
Questions in other subjects:




Mathematics, 23.10.2019 06:50



Biology, 23.10.2019 06:50

Mathematics, 23.10.2019 06:50

Mathematics, 23.10.2019 06:50

Mathematics, 23.10.2019 06:50

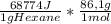 = -5,921x10⁶J/mol
= -5,921x10⁶J/mol

