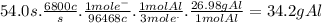Chemistry, 28.11.2019 00:31 angeljaylyn123
In the hall-heroult process, a large electric current is passed through a solution of aluminum oxide (a12o 3) dissolved in molten cryolite (na3aif6) ,resulting in the reduction of the ai�3 to pure aluminum. suppose a current of 6800.a is passed through a hall-heroult cell for 54.0 seconds. calculate the mass of pure aluminum produced. be sure your answer has a unit symbol and the correct number of significant digits.

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 04:00, eborkins
Seltzer water is created by placing water under pressure with carbon dioxide gas. which of the following statements best describe seltzer water: a. the solution will be slightly acidic b. the solution will be slightly basic. the solution will be strongly acidic. d. the solution will be strongly basic. e. the solution will be neutral
Answers: 3


Chemistry, 23.06.2019 03:30, cupcake3103670
Name 3 types of energy you see being used as you look around a classroom
Answers: 1
You know the right answer?
In the hall-heroult process, a large electric current is passed through a solution of aluminum oxide...
Questions in other subjects:







Chemistry, 11.10.2019 04:40

Chemistry, 11.10.2019 04:40