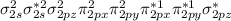Chemistry, 27.11.2019 23:31 zachthomas024
Which of the following statements relating to molecular orbital (mo) theory is incorrect? a. combination of two 2p orbitals may result in either latex: \sigma σ or latex: \pi π mos. b. combination of two atomic orbitals produces one bonding and one antibonding mo. c. a bonding mo is lower in energy than the two atomic orbitals from which it is formed. d. in a stable molecule having an even number of electrons, all electrons must be paired. e. a species with a bond order of zero will not be stable.

Answers: 2


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 03:30, asianaenaeh
Select the correct answer. when carbon dioxide dissolves in water, it sometimes reacts with water to form carbonic acid as in this balanced equation: co2 + h2o → h2co3. if 495 milliliters of carbon dioxide at 25°c and 101.3 kilopascals reacts with excess water, what is the theoretical yield of carbonic acid? use the periodic table and the ideal gas resource a. 0.889 g b. 1.10g c. 1.27g d. 2.02g what's the answer! quick!
Answers: 1

Chemistry, 22.06.2019 12:30, kingbot350
How many grams of magnesium metal will react completely with 8.3 liters of 5.5m hcl? show all work
Answers: 1

Chemistry, 22.06.2019 18:30, madmatt873
What volume of a 0.0606 m solution of strontium bromide is needed to obtain 0.340 mol of the compound? question 42 options: a)5.61 l b) 3.4 l c) 600 ml d) 1 l e) 178 ml
Answers: 1
You know the right answer?
Which of the following statements relating to molecular orbital (mo) theory is incorrect? a. combina...
Questions in other subjects:


Mathematics, 12.12.2019 00:31



History, 12.12.2019 00:31

Mathematics, 12.12.2019 00:31

Biology, 12.12.2019 00:31