Chemistry, 27.11.2019 22:31 harleypage308
Suppose 1.00 mol superheated ice melts to liquid water at 25°c. assume the specific heats of ice and liquid water have the same value and are independent of temperature. the enthalpy change for the melting of ice at 0°c is 6007 j mol21. calculate dh, dssys, and dg for this process.

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 21:00, rah45
Which of these is an example of pseudoscience? a) predicting the time of sunrise based on data on position of earth b) predicting the date of the moon phases based on data on position of earth c) predicting eclipses based on the position of the sun and the moon d) predicting future events in a person's life based on the position of the moon
Answers: 1


Chemistry, 23.06.2019 03:00, sharondacarruth1656
Is it safe to take 450mg of diphenhydramine hydrochloride?
Answers: 1

Chemistry, 23.06.2019 09:30, Nextlevel3
Where are the noble gases located in the periodic table? a. in the center b. on the left side c. in the upper right corner d. on the far right side
Answers: 1
You know the right answer?
Suppose 1.00 mol superheated ice melts to liquid water at 25°c. assume the specific heats of ice and...
Questions in other subjects:

Mathematics, 06.05.2020 08:35





Social Studies, 06.05.2020 08:35




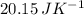
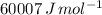
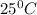
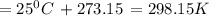
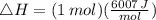
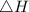 ) for the melting of 1.00 mole of ice at
) for the melting of 1.00 mole of ice at 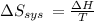
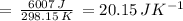
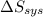 ) for the melting of 1.00 mole of ice at
) for the melting of 1.00 mole of ice at 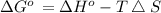
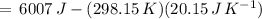
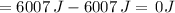
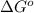 ) for the melting of 1.00 mole of ice at
) for the melting of 1.00 mole of ice at