
Chemistry, 27.11.2019 22:31 keishadawson
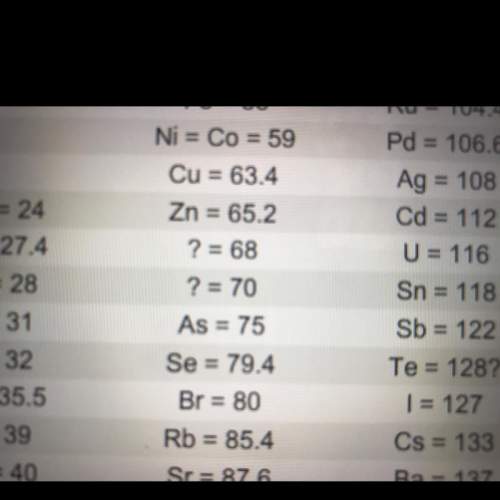
look at the two question marks between zinc (zn) and arsenic (as). at the time, no elements were known with atomic weights between 65.2 and 75. but mendeleev predicted that two elements must exist with atomic weights in this range.
what led mendeleev to predict that two undiscovered elements existed in that range?

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 18:20, datboyjulio21
Complete the table for ion charge based upon their losing or gaining electrons in the outer shell. (use the periodic table as necessary.) group most likely ionic charge # of valence electrons i +1 ii +2 iii +3 iv +4 or -4 v -3 vi -2 vii -1 viii 0
Answers: 2

Chemistry, 22.06.2019 06:10, gabriellestaleyga16
How many moles of gas are present if p=11 atm, v=12l, t=185k?
Answers: 1

Chemistry, 22.06.2019 12:00, shifaxoxoxo
What term is applied to a scientist who studies ancient life, including animal and plant fossils a. anthropologist b. dendroclimatologist c. geophysicist d. paleontologist
Answers: 2

Chemistry, 23.06.2019 01:30, mindofnyny
Which is an example of a highly unstable isotope that is often used in fission reactions?
Answers: 1
You know the right answer?
look at the two question marks between zinc (zn) and arsenic (as). at the time, no elements were kno...
Questions in other subjects:


History, 10.11.2020 21:20

Mathematics, 10.11.2020 21:20


Mathematics, 10.11.2020 21:20

History, 10.11.2020 21:20


English, 10.11.2020 21:20




