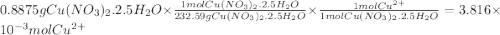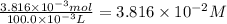Answers: 3


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 15:00, Dreambig85
Use examples from the article to explain one positive and one negative effect that chemistry has had on society
Answers: 2


You know the right answer?
Astock solution of cu2+(aq) was prepared by placing 0.8875 g of solid cu(no3)2∙2.5 h2o in a 100.0-ml...
Questions in other subjects:

Spanish, 28.06.2019 23:30

Mathematics, 28.06.2019 23:30


History, 28.06.2019 23:30


Mathematics, 28.06.2019 23:30

Physics, 28.06.2019 23:30

English, 28.06.2019 23:30


Geography, 28.06.2019 23:30