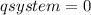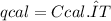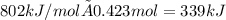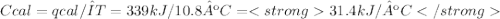Chemistry, 27.11.2019 07:31 stophendless9780
The heat capacity of a bomb calorimeter was determined by burning 6.79 g methane (energy of combustion 802 kj/mol ch4) in the bomb. the temperature changed by 10.8c. a. what is the heat capacity of the bomb? b. a 12.6-g sample of acetylene, c2h2, produced a temperature increase of 16.9c in the same calorimeter. what is the energy of combustion of acetylene (in kj/mol)?

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 03:30, fbillinton
In this chemical reaction, 325 grams of barium (ba) react completely. how many moles of lithium (li) are produced?
Answers: 1

Chemistry, 22.06.2019 10:40, trinityanne1738
Asolid that forms and separates from a liquid mixture is called
Answers: 2

Chemistry, 23.06.2019 04:00, hailey200127
What is the volume of 2.5 moles of nitrogen gas (n2)at standard temperature and pressure (stp)?
Answers: 1

Chemistry, 23.06.2019 13:00, Crxymia
How long could you survive without electricity? what parts of your life would be affected by loss of electricity? should you prepare for an electricity outage, and if so, how would you prepare? what backup system could your family or community install to generate limited amounts of electricity during an outage? how does this system create an electric force field and generate electric current?
Answers: 2
You know the right answer?
The heat capacity of a bomb calorimeter was determined by burning 6.79 g methane (energy of combusti...
Questions in other subjects:


Chemistry, 22.01.2020 00:31


History, 22.01.2020 00:31



Mathematics, 22.01.2020 00:31

English, 22.01.2020 00:31