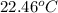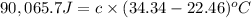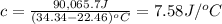Chemistry, 27.11.2019 06:31 lineaeriksen
A2.15g sample of benzene (c_6h_6) is burned in a bomb calorimeter, and the temperature rises from 22.46 degree c to 34.34 degree c. calculate the heat capacity of the bomb calorimeter. note the following thermochemical equation: c_6h_6(i) + 15/2 o_2 (g) rightarrow 6co_2 (g) + 3h_2o (g) delta h degree = -3267.5 kj

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 06:00, Kjswagout5052
In an investigation that uses the scientific method, which step immediately follows making a hypothesis? o summarizing the results o asking a question o making observations designing an experiment mark this and retum save and exit next submit
Answers: 2


Chemistry, 22.06.2019 10:00, halohero7
3. how much energy in joules is required to evaporate .0005 kg of liquid ammonia to vapor at the same temperature? 4. how much energy ( in megajoules ) is given up by .75 kg of water at 0c when it freezes to form ice at 0c? 5. explain how heat works between and at critical temperatures?
Answers: 2

Chemistry, 22.06.2019 14:30, lorrainelopez
The three types is stress that act on earths rocks are compression, tension, and
Answers: 1
You know the right answer?
A2.15g sample of benzene (c_6h_6) is burned in a bomb calorimeter, and the temperature rises from 22...
Questions in other subjects:





Mathematics, 25.04.2021 19:40

Mathematics, 25.04.2021 19:40


English, 25.04.2021 19:40

Mathematics, 25.04.2021 19:40

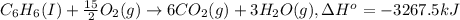
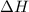 = enthalpy change = -3267.5 kJ/mol
= enthalpy change = -3267.5 kJ/mol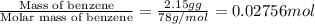
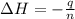
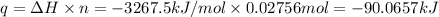
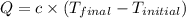
 = final temperature =
= final temperature = 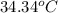
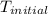 = initial temperature =
= initial temperature =