

Answers: 2


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 12:30, ethanw8973
If 22.5 liters of oxygen reacted with excess of hydrogen, how many liters of water vapor could be produced?
Answers: 3

Chemistry, 22.06.2019 19:00, hmontalvo22
How many moles are contained in 5.6 l of h2 at stp
Answers: 3

Chemistry, 22.06.2019 21:40, k3rbycalilung
Tooth enamel consists mainly of the mineral calcium hydroxyapatite, ca_10(po_4)_6(oh)_2. trace elements in teeth of archaeological specimens provide anthropologist with clues about diet and diseases of ancient people. students at hamline university measured strontium in enamel from extracted wisdom teeth by atomic absorption spectroscopy. solutions with a constant total volume of 10.0 ml contained 0.726 mg of dissolved tooth enamel plus variable concentrations of added sr. added sr find the concentration of sr in the 10 ml sample solution in parts per billion = ng/ml. find the concentration of sr in tooth enamel in parts per million = mu g/g.
Answers: 2
You know the right answer?
A35.00 ml sample of mg(no3)2 was diluted with water to 62.50 ml. a 20.00 ml sample of the dilute sol...
Questions in other subjects:


Mathematics, 22.07.2019 01:10


Mathematics, 22.07.2019 01:10






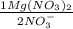 = 0,150M Mg(NO₃)₂
= 0,150M Mg(NO₃)₂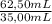 = 0,268M
= 0,268M

