
Chemistry, 07.10.2019 01:00 tiffanybrown703
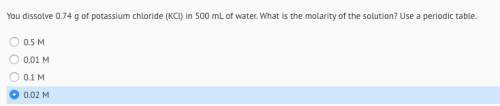
You dissolve 0.74 g of potassium chloride (kcl) in 500 ml of water. what is the molarity of the solution?

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 00:00, brookemcelhaney
Which of the following methods uses the decay of atomic particles in an object to find its exact age? a. fossil dating b. geologic dating c. radioactive dating d. relative dating
Answers: 1


Chemistry, 22.06.2019 19:30, youngdelvin123
Acetylene gas c2h2 undergoes combustion to produce carbon dioxide and water vapor how many grams of water are produced by the same amount of c2h2?
Answers: 2

Chemistry, 23.06.2019 01:00, kaykardash
Which polymers are most closely related? a. protein and nucleic acids b. cellulose and starch c. nucleic acids and starch d. nucleic acids and cellulose
Answers: 2
You know the right answer?
You dissolve 0.74 g of potassium chloride (kcl) in 500 ml of water. what is the molarity of the solu...
Questions in other subjects:

Business, 10.12.2020 21:10

Mathematics, 10.12.2020 21:10

History, 10.12.2020 21:10

Mathematics, 10.12.2020 21:10


Mathematics, 10.12.2020 21:10


Arts, 10.12.2020 21:10

Mathematics, 10.12.2020 21:10



