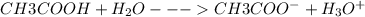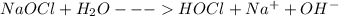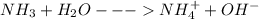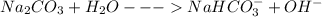Chemistry, 27.11.2019 03:31 xMABRYx1991
Write net brønsted equations that show the acidic or basic nature of the following solutions in water. for polyprotic species (vitamin c, lemon juice, and washing soda), show only one proton transfer. remember that spectator ions are not included. (use the lowest possible coefficients. omit states-of-matter in your answer.)
(a) vinegar (acetic acid, hc2h3o2)
(b) bleach (sodium hypochlorite, naocl)
(c) ammonia (nh3)
(d) vitamin c (ascorbic acid, h2c6h6o6)
(e) lemon juice (citric acid, h3c6h5o7)
(f) washing soda (sodium carbonate, na2co3)

Answers: 2


Other questions on the subject: Chemistry



Chemistry, 22.06.2019 16:00, julesperez22
Click the button that shows the correct relationship of the electron affinities of the elements sodium and phosphorus. sodium’s electron affinity value is more negative than the electron affinity value of phosphorus. phosphorus’ electron affinity value is more negative than the electron affinity value of sodium. this information cannot be determined using the periodic table. answer is b on e2020.
Answers: 3
You know the right answer?
Write net brønsted equations that show the acidic or basic nature of the following solutions in wate...
Questions in other subjects:

Law, 04.12.2020 14:00



Mathematics, 04.12.2020 14:00

Mathematics, 04.12.2020 14:00

History, 04.12.2020 14:00




Chemistry, 04.12.2020 14:00