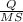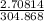If 50.00 ml of 1.05 m sodium hydroxide is added to 25.00 ml of 1.88 m hydrochloric acid, with both solutions originally at 24.66°c, what will be the final solution temperature? (assume that no heat is lost to the surrounding air and that the solution produced in the neutralization reaction has a density of 1.02 g/ml and a specific heat of 3.98 jg⁻¹°c⁻¹.)

Answers: 2


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 20:30, jaydenbrock
Identify the correct mole ratio for each substance. sodium chloride (nacl) na: cl = 1: ammonium nitrate (nhno) h: o = 4:
Answers: 1

Chemistry, 22.06.2019 22:00, choatefarmsus
Does the number of ions in solution increase, decrease, or remain constant? it continuously decreases. it continuously increases. it decreases at first, then increases. it increases at first, then decreases.
Answers: 3
You know the right answer?
If 50.00 ml of 1.05 m sodium hydroxide is added to 25.00 ml of 1.88 m hydrochloric acid, with both s...
Questions in other subjects:




Social Studies, 19.03.2020 18:47



Mathematics, 19.03.2020 18:47


Mathematics, 19.03.2020 18:47

Mathematics, 19.03.2020 18:47