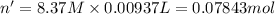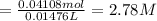Calculate the concentration of acid(or base) remaining in solution when 5.39ml of 6.93m hno3 is added to 9.37ml of 8.37m naoh. note the final volume is the sum of two added volumes. which of the following statement is true for the solution after mixing? a) naoh is in excess overhno3b)hno3 is in excess over naohc)hno3 and naoh are exactly balanced. what is the concentration of the excess naoh (or hno3) you indicated above?

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 15:50, leannamat2106
If the mass of the products measured 120g what would the mass of the reactants a. 30g b. 60g c. 120g d. 240g
Answers: 1

Chemistry, 22.06.2019 12:00, WinterStrikesBack
Solutions of sodium carbonate and silver nitrate react to form solid silver carbonate and a solution of sodium nitrate. a solution containing 3.50 g of sodium carbonate is mixed with one containing 5.00 g of silver nitrate. how many grams of sodium carbonate, silver nitrate, silver carbonate, and sodium nitrate are present after the reaction is complete?
Answers: 2


Chemistry, 22.06.2019 20:00, bettybales1986
Glucose (c6h12o6) is an important biological molecule. (round the answer to nearest hundredth.) what is the percent by mass of carbon in glucose?
Answers: 2
You know the right answer?
Calculate the concentration of acid(or base) remaining in solution when 5.39ml of 6.93m hno3 is adde...
Questions in other subjects:

Mathematics, 08.10.2019 11:20

Mathematics, 08.10.2019 11:20

History, 08.10.2019 11:20

Geography, 08.10.2019 11:20



Mathematics, 08.10.2019 11:20

Mathematics, 08.10.2019 11:20


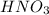 .
.