
Chemistry, 27.11.2019 02:31 merrymary3000
The balanced equation for the reaction of bromate ion with bromide in acidic solution is  at a particular instant in time, the rate of disappearance of br– is 2.0 x 10⁻³ mol/l • s. what is the rate of appearance of br₂ at the same instant?
at a particular instant in time, the rate of disappearance of br– is 2.0 x 10⁻³ mol/l • s. what is the rate of appearance of br₂ at the same instant?

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 00:30, natalie1755
Butadiene undergoes a reaction at a certain temperature in the gas phase as follows: 2c4h6(g) --> c8h12(g) the following data were collected for this reaction: time (min) [c4h6] (m) 0 0.36 15 0.30 30 0.25 48 0.19 75 0. determine the order of the reaction and the rate constant. 1st order and k = 4.3x10 -4 s-1 1st order and k = 2.3x10-4 s-1 2nd order and k = 4.3x10-4 s-1 2nd order and k = 2.3x10-4 s-1 zero and k = 4.3x10-4 s-1
Answers: 3

Chemistry, 22.06.2019 04:00, nothingworksoutforme
The image shows a process that releases nuclear energy which statement best identifies the process shown the process must be fusion because energy is released the process must be fusion because of have your nucleus formed a smaller nuclei the process must be fission because a large nucleus breaks into smaller nuclei the process must be fission because neutrons are formed
Answers: 1

Chemistry, 22.06.2019 06:00, coopera1744
Find the mass in grams of 1.37x1020 particles of h3po4
Answers: 2

You know the right answer?
The balanced equation for the reaction of bromate ion with bromide in acidic solution is [tex]bro^-...
Questions in other subjects:

German, 04.04.2020 13:01









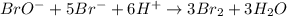
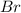 :
: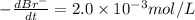
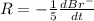
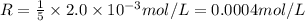
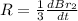
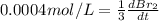
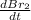 =3\times 0.0004 mol/L=0.0012 mol/L[/tex]
=3\times 0.0004 mol/L=0.0012 mol/L[/tex]

