Chemistry, 27.11.2019 01:31 lattimorekeonna1
If 60.0 grams of carbonic acid are sealed in a 2.00 l soda bottle at room temperature (298 k) and decompose completely via the equation below, what would be the final pressure of carbon dioxide assuming it had the full 2.00 l in which to expand? h₂co₃(aq) → h₂o(l) + co₂(g)

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 04:30, terrancebest
Which is a chemical property of iron? a. it forms iron oxide (rust) when exposed to moisture and air. b. it is a gray–black metal that is hard to the touch. c. it has a melting point of 2795°f (1536°c). d. it is a good conductor of heat
Answers: 2

Chemistry, 22.06.2019 13:00, carlinryan
16. why must the number of electrons lost equal the number of electrons gained in every redox reaction? use 3 – 4 sentences in your own words to address this question. 18. what type of radiation is emitted when chromium-51 decays into manganese-51? show the nuclear equation that leads you to this answer. 19. a radioactive nucleus alpha decays to yield a sodium-24 nucleus in 14.8 hours. what was the identity of the original nucleus? show the nuclear equation that leads you to this answer.
Answers: 2

Chemistry, 22.06.2019 17:00, abbygailgo674
How can a give a full method for the experiment of separating sand from water by filtration? 1-materials 2-steps 3-conclusion also for water and salt separated by the evaporation or distillation process
Answers: 1

Chemistry, 22.06.2019 18:30, tanviknawale
Which sample at stp has the same number of atoms as 18 liters of ne at stp
Answers: 1
You know the right answer?
If 60.0 grams of carbonic acid are sealed in a 2.00 l soda bottle at room temperature (298 k) and de...
Questions in other subjects:



Mathematics, 10.09.2021 01:30

Mathematics, 10.09.2021 01:30

History, 10.09.2021 01:30


Mathematics, 10.09.2021 01:30


History, 10.09.2021 01:30

Biology, 10.09.2021 01:30

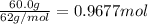
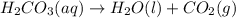
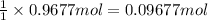 of carbon dioxide
of carbon dioxide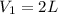
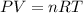 (ideal gas law)
(ideal gas law)