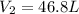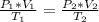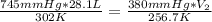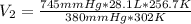Chemistry, 27.11.2019 00:31 happysage12
Aweather balloon is inflated to a volume of 28.1 l at a pressure of 745 mmhg and a temperature of 29.0 ∘c. the balloon rises in the atmosphere to an altitude where the pressure is 380. mmhg and the temperature is -16.3 ∘c. assuming the balloon can freely expand, calculate the volume of the balloon at this altitude.

Answers: 2


Other questions on the subject: Chemistry



Chemistry, 22.06.2019 14:50, jonmorton159
Consider the following multistep reaction: a b→ab(slow) a ab→a2b(fast)−−−−−−−−−−−−−−−−− 2a b→a2b(overall) based on this mechanism, determine the rate law for the overall reaction. express your answer in standard masteringchemistry format. for example, if the rate law is k[a]3[b]2 type k*[a]^3*[b]^2
Answers: 3

Chemistry, 22.06.2019 21:30, jpimentel2021
Under which circumstances are kp and kc equal for the reaction aa(g)+bb(g)⇌cc(g)+dd(g)?
Answers: 2
You know the right answer?
Aweather balloon is inflated to a volume of 28.1 l at a pressure of 745 mmhg and a temperature of 29...
Questions in other subjects:

Mathematics, 20.11.2020 17:20


Mathematics, 20.11.2020 17:20

English, 20.11.2020 17:20

Physics, 20.11.2020 17:20

Mathematics, 20.11.2020 17:20

Mathematics, 20.11.2020 17:20