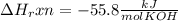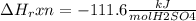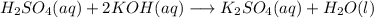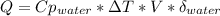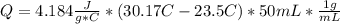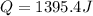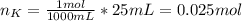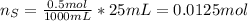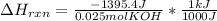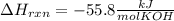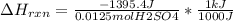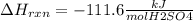When 25.0 ml of 0.500 m h2so4 is added to 25.00 mk of 1.00 m koh in a coffee-cup calorimeter at 23.50◦ c, the temperature rises to 30.17◦ c. calculate dhrxn for this reaction. (assume that the density of water of and specific heat capacity of the solution are the same as for pure water). ( –55.8 kj/mol koh, –112 kj/mol h2so4)

Answers: 1


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 00:40, eamccoy1
Base your answer on the information below and on your knowledge of chemistry. nitrogen dioxide, no2, is a dark brown gas that is used to make nitric acid and to bleach flour. nitrogen dioxide has a boiling point of 294 k at 101.3 kpa. in a rigid cylinder with a movable piston, nitrogen dioxide can be in equilibrium with colorless dinitrogen tetroxide, n2o4. this equilibrium is represented by the equation below. 2no2(g) n2o4(g) + 58kj at standard pressure, compare the strength of intermolecular forces in no2(g) to the strength of intermolecular forces in n2(g).
Answers: 2


Chemistry, 23.06.2019 01:00, tjeffers90028
Iron (fe) reacts with copper sulfate (cuso4) to form iron (ii) sulfate. in this reaction, cu2+ gains electrons to form cu. which statement is true about this reaction? fe(s) + cuso4(aq) → feso4(aq) + cu(s)
Answers: 3
You know the right answer?
When 25.0 ml of 0.500 m h2so4 is added to 25.00 mk of 1.00 m koh in a coffee-cup calorimeter at 23.5...
Questions in other subjects:


Chemistry, 01.07.2020 15:01


Physics, 01.07.2020 15:01


Mathematics, 01.07.2020 15:01