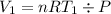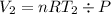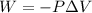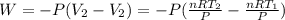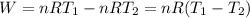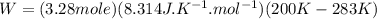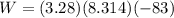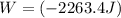Chemistry, 26.11.2019 23:31 parkerwallace04
Acylinder with a moveable piston contains 92g of nitrogen. the external pressure is constant at 1.00 atm. the initial temperature is 200k. when the temperature is increased by 83 k, by taking it out of the freezer, the volume will increase, according to the ideal gas law. calculate the work for this process. express your answer in j.

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 05:30, fgcherubin
What happens to the atomic radius when an elctron is lost
Answers: 1

Chemistry, 22.06.2019 09:00, 2024cynthiatercero
Which two scientific disciplines are bridged by pharmaceutical drugs? chemistry and forensics chemistry and medicine biology and forensics biology and criminology
Answers: 3

Chemistry, 22.06.2019 19:00, montgomerykarloxc24x
Convert the temperature of dry ice, –77 ∞c, into degrees fahrenheit and kelvin.
Answers: 2

Chemistry, 23.06.2019 01:30, Thunderalesis7855
Concentrations expressed as a percent by mass are useful when the solute is a a. liquid b. gas c. solid
Answers: 1
You know the right answer?
Acylinder with a moveable piston contains 92g of nitrogen. the external pressure is constant at 1.00...
Questions in other subjects:



Geography, 12.01.2021 19:00

Mathematics, 12.01.2021 19:00

English, 12.01.2021 19:00


World Languages, 12.01.2021 19:00

Chemistry, 12.01.2021 19:00

Physics, 12.01.2021 19:00