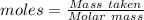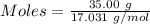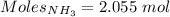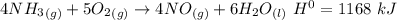Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 01:30, montoyaricardo3550
Aroller coaster car is traveling down a track at 22 m/s. the car has a mass of 2000 kg. what is the kinetic energy of the car? a) 22,000 j b) 968,000 j c) 484,000 j d) 44,000 j
Answers: 2


Chemistry, 22.06.2019 11:00, 21villalobosjabez
Which type of fossil does this image depict?
Answers: 1

Chemistry, 22.06.2019 19:30, amandamiro05
Helium decays to form lithium. which equation correctly describes this decay?
Answers: 2
You know the right answer?
How much heat is absorbed/released when 35.00 g of nh3(g) reacts in the presence of excess o2(g) to...
Questions in other subjects:


History, 23.10.2019 06:00




Mathematics, 23.10.2019 06:00

History, 23.10.2019 06:00



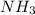 :-
:-