Chemistry, 26.11.2019 21:31 jiboyajordan2069
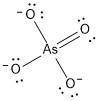
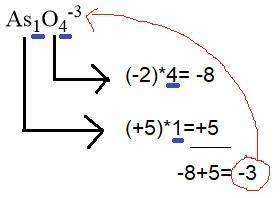
Draw a lewis structure for the resonance form of aso4^-3, with the lowest possible formal charges. include any nonzero formal charges and lone pair electrons in the structure.
-what is the oxidation number of as
-what is the oxidation number of o

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 01:30, adrian08022
When an object falls through the air and encounters air resistance its overall speed will be than if it had not encountered air resistance? (one word answer)
Answers: 2

Chemistry, 22.06.2019 03:30, acaciacoats
The atomic radius of sodium is 186 pm and of chlorine is 100 pm. the ionic radius for na+ is 102 pm and for cl– is 181 pm. in going from na to cl in period 3, why does the atomic radius decrease while the ionic radius increases? a. the inner electrons in the sodium cation shield its valence electrons more effectively than the inner electrons in the chloride anion do. b. the inner electrons shield the valence electrons more effectively in the chlorine atom than in the chloride anion. c. the outermost electrons in chloride experience a smaller effective nuclear charge than those in the sodium cation do. d. the outermost electrons in chloride experience a larger effective nuclear charge than those in the sodium cation do. e. monatomic ions are bigger than the atoms from which they are formed.
Answers: 2


Chemistry, 22.06.2019 10:00, valdezlizbeth6652
Why is carbon ideal for making different compounds?
Answers: 2
You know the right answer?
Draw a lewis structure for the resonance form of aso4^-3, with the lowest possible formal charges. i...
Questions in other subjects:

English, 26.05.2020 02:03

Mathematics, 26.05.2020 02:03


Mathematics, 26.05.2020 02:03

Biology, 26.05.2020 02:03

English, 26.05.2020 02:03



History, 26.05.2020 02:03

Mathematics, 26.05.2020 02:03