Chemistry, 26.11.2019 21:31 Ciarrathereal
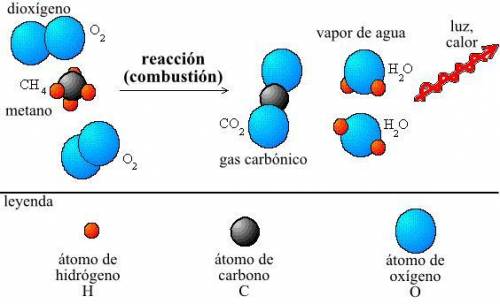
Achemist measures the energy change δh during the following reaction: c3h8 (g) +5o2 (g) →3co2 (g) +4h2o (l) =δh−2220.kj use the information to answer the following questions.
this reaction
endothermic.
exothermic.
suppose
81.0g
of
c3h8
react.
will any heat be released or absorbed?
yes, absorbed.
yes, released.
no.
if you said heat will be released or absorbed in the second part of this question, calculate how much heat will be released or absorbed.
kj
round your answer to
3
significant digits.

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 07:00, maryjane8872
Which set of characteristics best describes igneous rock? a) largest type of rock, made of organic matter, hardest type of rock b) least abundant type of rock, made of other rocks, made mostly of minerals c) found on all continents, contains wavy bands of stripes, contains fossils d) most abundant type in earth's crust, made of magma/lava, contains no fossils
Answers: 1

Chemistry, 22.06.2019 11:00, snowprincess99447
Which statement is true about hcl? (5 points) select one: a. it is a salt because it increases the concentration of metallic ions. b. it is a salt because it is formed by the reaction of an acid and a base. c. it is an acid because it increases the concentration of hydroxyl ions. d. it is an acid because it increases the concentration of hydronium ions.
Answers: 1


Chemistry, 22.06.2019 22:30, brianna5626
How do limiting factors most affect population size? ostop population growthrestrict population growthincrease population sizeresult in positive impactso
Answers: 1
You know the right answer?
Achemist measures the energy change δh during the following reaction: c3h8 (g) +5o2 (g) →3co2 (g) +...
Questions in other subjects:



Social Studies, 19.06.2020 03:57


Mathematics, 19.06.2020 03:57

Mathematics, 19.06.2020 03:57


Mathematics, 19.06.2020 03:57

Mathematics, 19.06.2020 03:57

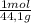 ×
×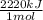 = 4,08x10³ kJ
= 4,08x10³ kJ