
Chemistry, 26.11.2019 21:31 senituliii
Sulfur has an atomic mass of 32 u and avogadro's number is 6 × 1023 formula units/mole. the number of sulfur atoms in 1 g of sulfur is:

Answers: 3


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 03:00, cheesecake1919
In the 1800s, one of the statements in john dalton's atomic theory was that atoms are indivisible. later experimental evidence led to the discovery of subatomic particles such as neutrons, electrons, and protons. what happened to the indivisible atom part of dalton's atomic theory, and why?
Answers: 3

Chemistry, 22.06.2019 06:00, josmanu235
Why is permeable soil best for plants that need a lot of drainage?
Answers: 1

Chemistry, 22.06.2019 13:00, wbrandi118
Using the thermodynamic information in the aleks data tab, calculate the standard reaction free energy of the following chemical reaction: →+p4o10s6h2ol4h3po4s round your answer to zero decimal places.
Answers: 3
You know the right answer?
Sulfur has an atomic mass of 32 u and avogadro's number is 6 × 1023 formula units/mole. the number o...
Questions in other subjects:


History, 10.12.2020 20:50

Mathematics, 10.12.2020 20:50


Mathematics, 10.12.2020 20:50


Mathematics, 10.12.2020 20:50

English, 10.12.2020 20:50


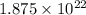 atoms
atoms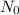 .
.
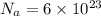
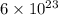 of atoms
of atoms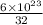 of atoms
of atoms

