
Chemistry, 26.11.2019 21:31 AshlynPlayz45
Consider the following reaction at constant pressure. use the information provided below to determine the value of δs at 473 k. predict whether or not this reaction will be spontaneous at this temperature.
4nh3 (g) + 3o2 (g) → 2n2 (g) + 6h2o (g) δh = –1267 kj

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 04:30, EinsteinBro
Which statement best describes the relationship between period and frequency of light waves? a) in wave b the period increases and the frequency decreases from wave a. b) in wave a the period increases and the frequency decreases from wave b. c) in wave b the period is shorter and the frequency is greater than in wave a. d) in wave a the period is shorter and the frequency is greater than in wave b.
Answers: 1

Chemistry, 22.06.2019 07:00, coolkid2041
Calculate the number of moles of ethane in 100 grams
Answers: 3


Chemistry, 22.06.2019 12:30, hayleyconsole
Nebulae are enormous clouds in outer space. they are made mostly of hydrogen gas, helium gas, and dust. some nebulae glow brightly, while others do not. the stars that people see are huge, bright balls of glowing gas. they are made mostly of hydrogen and helium. which statement correctly describes other ways in which nebulae and stars are different? a. stars can form inside a nebula but a nebula can never be produced by any star. b. a star always has a higher density than a nebula. c. stars can never form inside a nebula but a nebula can be produced by any star. d. a nebula always has a higher density than a star.
Answers: 3
You know the right answer?
Consider the following reaction at constant pressure. use the information provided below to determin...
Questions in other subjects:










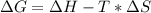
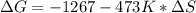 :
: ![\Delta S0[/tex}Back to this expression: [tex]\Delta G= -1267 - 473 K* \Delta S](/tpl/images/0392/0613/808f0.png)



