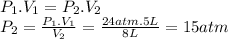Two tanks (tank a and tank b) of gas are connected by a closed valve. tank a is 5 liters and contains o2 gas at a pressure of 24 atm. tank b is 3 liters and contains n2 gas at a pressure of 32 atm. both tanks are held at the same temperature. the valve between the two tanks is opened and the gases are allowed to mix. after the gases have had time to mix, what is the partial pressure of the oxygen gas?

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 03:30, jabper5522
At a temperature of 393 k, the temperature of a sample of nitrogen is 1.07 atm what will the pressure be at a temperature of 478 k
Answers: 1

Chemistry, 22.06.2019 11:50, hadwell34
Which of the following statements about hybrid orbitals is or are true? choose all that apply. choose all that apply. under sp2 hybridization, the large lobes point to the vertices of an equilateral triangle. after an atom undergoes sp hybridization there is one unhybridized p orbital on the atom. the angle between the large lobes of sp3 hybrids is 109.5∘
Answers: 2

You know the right answer?
Two tanks (tank a and tank b) of gas are connected by a closed valve. tank a is 5 liters and contain...
Questions in other subjects:


Mathematics, 23.01.2020 07:31

Biology, 23.01.2020 07:31

Business, 23.01.2020 07:31



Mathematics, 23.01.2020 07:31


History, 23.01.2020 07:31

Mathematics, 23.01.2020 07:31