Chemistry, 26.11.2019 17:31 lanettejohnson355
Using the following data, determine the standard cell potential e^o cell for the electrochemical cell constructed using the following reaction, where zinc is the anode and lead is the cathode.
zn(s) + pb2+(aq) -> zn2+(aq) + pb(s)
half-reaction: standard reduction potential:
zn2+(aq) + 2e- -> zn(s)= -0.763
pb2+(aq) + 2e- -> pb(s)= -0.126
a. -0.889 v
b. +0.889 v
c. +0.637 v
d. +1.274 v
e. -0.637 v

Answers: 2


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 10:00, emfranco1
Ill give brainiestif one neutron initiates a fission event that produces two neutrons in the products, how many new reactions can now be initiated? if each of the neutrons produced in the first fission event then initiates a fission event that produces one neutron in the products, how many new reactions can now be initiated by each neutron? how many neutrons in total were produced by the two fission events described?
Answers: 2

Chemistry, 22.06.2019 17:00, jazmine8194
Reduction is a reaction which results in a in electrons and a in positive charge of the atom or ion 1) a- loss 1) b- gain 2) a-increase 2) b-decrease
Answers: 1

Chemistry, 22.06.2019 18:00, jeepjose58
What amount of heat is exchanged when 106.2 grams of substance y goes from a liquid at 35 degrees celsius to a solid at the same temperature? melting point of substance y = 35 degrees c; δhvaporization = 3.67 j/mol; δhfusion = 3.30 j/mol. mwsubstance y = 28.22 g/mol. −12.4 j −3.51 x 102 j 1.24 x 101 j 351 j
Answers: 1
You know the right answer?
Using the following data, determine the standard cell potential e^o cell for the electrochemical cel...
Questions in other subjects:

Social Studies, 22.09.2019 01:00

Mathematics, 22.09.2019 01:00

Mathematics, 22.09.2019 01:00





Health, 22.09.2019 01:00


Biology, 22.09.2019 01:00

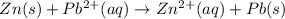
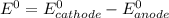
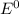 are standard reduction potentials.
are standard reduction potentials.
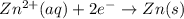 = -0.763
= -0.763
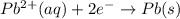 = -0.126
= -0.126![E^0_{[Zn^{2+}/Zn]}=-0.763V](/tpl/images/0391/6528/6b929.png)
![E^0_{[Pb^{2+}/Pb]}=-0.126V](/tpl/images/0391/6528/96123.png)
![E^0=E^0_{[Pb^{2+}/Pb]}- E^0_{[Zn^{2+}/Zn]}](/tpl/images/0391/6528/a01eb.png)