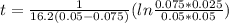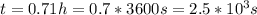a second- order reaction of the type a + b --> p was carried out in a solution that was initially 0.075 mol dm^-3 in a and 0.050 mol dm^-3 in b. after 1.0 h the concentration of a had fallen to 0.020 mol dm^-3. a) calculate the rate constant. b) solve for the half- life of each of the reactants.
hint: answers are a) 16.2 dm^3/mol*h
b) 5.1 × 10^3 s, 2.1 × 10^3 s

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 18:20, ineedhelp773
Which statement accurately describes the relationship between air pressure, air density, or altitude? as altitude increases, pressure increases. as altitude increases, air density increases. air pressure and density are lowest at sea level. denser air exerts more pressure than less dense air.
Answers: 2

Chemistry, 22.06.2019 01:00, chrisxxxrv24
What are the variables in gay-lussac’s law? pressure and volume pressure, temperature, and volume pressure and temperature volume, temperature, and moles of gas
Answers: 1


Chemistry, 22.06.2019 18:30, robjaykay
The famous scientist galileo galilei did several experiments with sloping planes, which he rolled metal balls down so that he could study motion. by changing the slope, he could study how the speed at which the ball rolled was affected. what was the independent variable in galileo's experiment? a. the speed of the ball b. the slope of the plane c. whether the ball moved d. what the ball was made of
Answers: 2
You know the right answer?
a second- order reaction of the type a + b --> p was carried out in a solution that was initially...
Questions in other subjects:

History, 02.12.2019 19:31

Chemistry, 02.12.2019 19:31


English, 02.12.2019 19:31



English, 02.12.2019 19:31

Mathematics, 02.12.2019 19:31


Mathematics, 02.12.2019 19:31


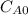 and
and 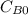 are the inital concentrations and x the concentration reacted at time t, so
are the inital concentrations and x the concentration reacted at time t, so 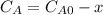 and
and 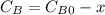 and the rate at time t is written as:
and the rate at time t is written as:
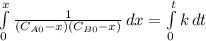

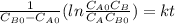
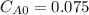 ,
,  ,
,  , it implies that the quantity reacted, x, is 0.03 and
, it implies that the quantity reacted, x, is 0.03 and  . Then, the value of k would be
. Then, the value of k would be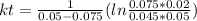
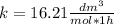
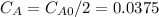 so
so  , k=16.2 and the same initial concentrations. Replacing in the equation
, k=16.2 and the same initial concentrations. Replacing in the equation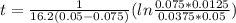

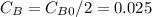 so
so  , k=16.2 and the same initial concentrations. Replacing in the equation
, k=16.2 and the same initial concentrations. Replacing in the equation