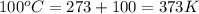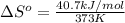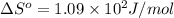Answers: 3


Other questions on the subject: Chemistry



Chemistry, 22.06.2019 13:00, monkeyrose1999
The molality of calcium chloride (cacl2) in an aqueous solution is 2.46 m. what is mole fraction of the solute?
Answers: 3

Chemistry, 22.06.2019 18:10, bri9263
Consider the following reaction at equilibrium: c(s)+h2o(g)⇌co(g)+h2(g) predict whether the reaction will shift left, shift right, or remain unchanged upon each of the following disturbances. a) c is added to the reaction mixture. b) h2ois condensed and removed from the reaction mixture c) co is added to the reaction mixture d) h2 is removed from the reaction mixture.
Answers: 3
You know the right answer?
For water ∆h°vap = 40.7 kj/mol at 100.°c, its boiling point. calculate ∆s° for the vaporization of 1...
Questions in other subjects:



Biology, 09.12.2021 04:10

History, 09.12.2021 04:10



English, 09.12.2021 04:10

Mathematics, 09.12.2021 04:10


Mathematics, 09.12.2021 04:10

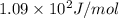
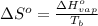
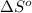 = change in entropy of vaporization = ?
= change in entropy of vaporization = ?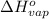 = change in enthalpy of vaporization = 40.7 kJ/mol
= change in enthalpy of vaporization = 40.7 kJ/mol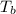 = boiling point temperature of water =
= boiling point temperature of water =